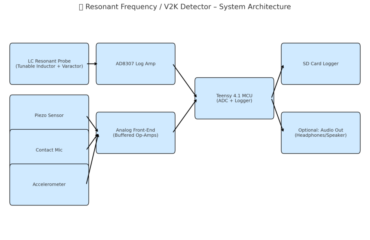
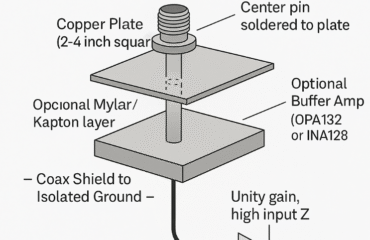
Capturing Skull Pulses & Knuckle Cracking Effects
🧠📡 Experimental Setup Design: Capturing Skull Pulses & Knuckle Cracking Effects
🔧 A. Sensor Modalities to Use
| Sensor Type | What It Captures | Placement |
|---|---|---|
| Piezoelectric film / contact mic | Vibration / pressure pulse | Knuckles, over ear, behind skull |
| MEMS accelerometers | Very fine skull movement (µg) | On skin near ear, cheekbone, knuckle |
| Laser Doppler Vibrometer | Surface motion (non-contact) | Pointed at skin near ear/temple |
| Electret microphone | Air-transmitted sound from knuckle | Near hand/head interface |
| Acoustic hydrophone | Pressure wave in gel or cavity | Embedded in test medium near head |
| Thermal camera (FLIR) | RF-induced heating patterns | Side of face / hand skin |
| Capacitive stretch sensor | Pressure expansion / movement | Across skin near ear or in knuckle joint |
| EEG/EMG pads | Neural/muscular activation | Temporalis, auricularis, hand muscles |
| Ultrasound mic | Airborne ultrasound >20kHz | Next to head, aimed at possible sources |
🧪 B. Specialized Materials & Test Rigs
Here are custom materials or mediums you can fabricate to act as “proxies” for skull/knuckle interaction and field detection:
✅ 1. Acrylic Skull Plate With Gel Overlay (Resonant Model)
- Use a thin, flat acrylic or polycarbonate sheet to simulate bone.
- Overlay with ultrasound gel, ballistics gel, or 3% agar gel to mimic soft tissue coupling.
- Embed piezo sensors, accelerometers, or hydrophones inside gel or at gel/acrylic interface.
- Press hand or knuckle against it as if it were the skull.
- Watch for vibration, impulse transmission, or delayed crack from interaction.
✅ 2. Layered Finger Proxy Material
- Mold a knuckle-sized block using:
- Outer layer of silicone skin substitute (DragonSkin, EcoFlex)
- Inner gelatin with embedded air bubbles
- A plastic or ceramic joint core
- This mimics tendon + bone + fluid.
- Attach piezo sensor inside.
- Place this in contact with your ear, see if pulses/cracks are transferred and detected.
✅ 3. Standing Wave Detection Panel
- Create a thin copper or mylar sheet on foam.
- Place against the head with knuckle pressed on top.
- Use piezo films or contact mics at intervals on the sheet to detect propagation and interference patterns from any field-induced or mechanical vibration.
- Could help isolate node/antinode zones if there’s a standing wave pattern.
👁️ C. Visualization Options
✅ 1. Laser Interferometry
- Use Laser Doppler Vibrometer or a low-power laser pointer + photodiode setup to detect motion.
- Aim at temple, forehead, or knuckle.
- Modulations in reflected laser intensity represent surface displacement (μm-scale).
- Can visualize 3-second pulses, micro-oscillations, or rhythmic skull expansion.
✅ 2. High-Speed Video with Reference Markers
- Place small reflective dots (stickers or talcum powder) on hand and head.
- Use 240+ FPS smartphone video or camera.
- Analyze motion between head and hand across time.
- Look for cyclical expansion (~3 sec) or microshifts in hand position.
✅ 3. IR Thermal Imaging
- Observe for thermal hotspots on ear/knuckle over time.
- RF pulses cause tiny but rapid tissue warming.
- Use thermal camera with sub-0.1°C resolution.
- Time-sync with audio or vibration capture.
📋 D. Suggested Test Configurations
Test 1: Knuckle-Over-Ear Direct Logging
- Piezo strip on knuckle + ear
- Audio + vibration logger
- Time-stamp: when hand goes on ear, when pulses/cracks start
Test 2: Acrylic Resonance Panel
- Simulated skull with gel + sensor array
- Apply knuckle to “ear zone”
- Try triggering field response using RF/ultrasound source
Test 3: Bone Conduction + Occlusion Simulation
- Use occluded ear + bone mic behind mastoid
- Apply different materials between hand and ear: metal, gel pad, fabric
- Measure vibration transmission differences
🧲 E. Shielding, Modulation, and Environmental Variables
| Variable | How to Manipulate | What It Proves |
|---|---|---|
| RF shielding | Faraday fabric, copper mesh | If RF is the source, effects will reduce |
| Acoustic damping | Foam or silicone layer | If sound, damping will reduce crack/pulse |
| Grounding | Wear grounding strap on hand/head | Tests for electrostatic or low-voltage coupling |
| Distance tests | Increase separation from devices | Helps triangulate or eliminate sources |
| Rotation tests | Turn head or body | Standing waves shift — shows spatial dependency |
🧪 Tools You May Need
- Piezo film vibration sensors (e.g., from TE Connectivity)
- Adafruit or SparkFun MEMS accelerometers
- Zoom H4N or Tascam recorder with contact mic input
- Thermal camera (Seek Thermal Pro or FLIR One)
- Ultrasonic mic (Dodotronic, Pettersson)
- Low-cost laser Doppler vibrometer (e.g., Polytec or homebrew kit)
- Ballistics gel, silicone molding kits (Smooth-On)
🧪 Matching Materials Based on Hypothesized Effect Types
| Hypothesis | What Needs to Be Matched | How to Simulate or Test |
|---|
🧠 1. Resonance-Based Interaction (RF or Acoustic)
Core Idea: Vibrations at a resonant frequency of bone, cartilage, or cavity are inducing mechanical or acoustic responses.
Properties to Match:
- Acoustic impedance (Z)
- Density (ρ) and elasticity (E) of tissue
- Geometry of ear canal + temporal bone
- Natural frequencies of hand/knuckle structures
Materials to Test:
- Acrylic (approx. bone stiffness)
- DragonSkin or EcoFlex (cartilage mimic)
- Air cavities inside gel (to mimic Helmholtz effect)
- 3D-printed bone simulants with known resonant modes
How to Simulate:
- Use frequency sweep (20 Hz to MHz) across ear/knuckle with sensors
- Use laser vibrometer to scan vibration modes
- Design variable-thickness resonators and tune gel properties to match skin/bone
📌 Math:
To match resonance, solve for natural frequency of a cavity:
f=c2πAVLf = \frac{c}{2\pi} \sqrt{\frac{A}{V L}}f=2πcVLA
Where:
- ccc = speed of sound in air
- AAA = cross-sectional area of canal
- VVV = volume of canal
- LLL = effective length of canal (with end correction)
You can build a Helmholtz test rig using this equation.
💥 2. Cavitation or Microbubble Collapse
Core Idea: Fluid or gel-like material (e.g. tissue, lymph, synovial fluid) is undergoing gas bubble collapse due to pressure shifts or vibration.
Properties to Match:
- Viscosity and surface tension of tissue fluid
- Gas solubility and degassing rate
- Tissue elasticity to contain bubble collapse energy
- Pressure differential over time (ΔP threshold for cavitation)
Materials to Test:
- Ballistic gel (10% to 20%) or agar-based gel
- Soft silicone with air bubble inclusions
- Degassed water-gel combinations inside soft skin simulant
How to Simulate:
- Use ultrasound probes to induce cavitation
- Use acoustic or RF pressure pulses and observe under high-speed camera or hydrophone
- Use transparent test blocks with bubbles and backlight to visualize micro-explosions
📌 Math:
For cavitation threshold (Blake threshold):
Pmin=Patm−2σRP_{min} = P_{atm} – \frac{2\sigma}{R}Pmin=Patm−R2σ
Where:
- σ\sigmaσ = surface tension
- RRR = bubble radius
- PminP_{min}Pmin = minimum pressure needed for cavitation
🧲 3. Electromechanical or RF Induced Pressure
Core Idea: EM signal (e.g., AM RF, pulsed microwave) is creating thermoelastic expansion or vibratory forces in localized regions of tissue.
Properties to Match:
- Dielectric constant (ε) and conductivity (σ) of tissue
- Permittivity/permeability
- Specific absorption rate (SAR) and thermal expansion coefficient
- Thermal time constant (to match 3s intervals)
Materials to Test:
- Saline-loaded agar gel
- Conductive hydrogels or carbon-doped silicone
- EM field-sensitive rubber/polymer sheets
- RF-absorbing foam with embedded thermal sensor or IR marker
How to Simulate:
- Expose phantom to modulated RF (e.g., 1.33 GHz AM at low duty cycle)
- Use thermal imaging + micro strain gauge
- Use Faraday cage control group
- Compare behavior with and without hand contact
📌 Math:
To estimate field coupling energy:
Q=σE2tQ = \sigma E^2 tQ=σE2t
Where:
- σ\sigmaσ = electrical conductivity
- EEE = electric field strength
- ttt = exposure time
And thermal expansion:
ΔL=αLΔT\Delta L = \alpha L \Delta TΔL=αLΔT
Where:
- α\alphaα = coefficient of thermal expansion
- LLL = length of tissue
- ΔT\Delta TΔT = temp change from RF
🪫 4. Neuromechanical or Myofascial Triggering (Nerve/Fascia Loop)
Core Idea: Signals (EM/US or mechanical) are stimulating nerve endings or fascia planes, causing twitches, tendon snaps, or echo responses.
Properties to Match:
- Elastic modulus of fascia (~0.5–1 MPa)
- Nerve conduction time (~3–10 ms + latency from stimulus)
- Fascia layering, slip planes, and trigger point zones
Materials to Test:
- Synthetic fascial planes (thin silicone sheets with lube layer)
- Conductive silicone over tendon models
- Use TENS pads to simulate localized EM nerve triggering
- Use ultrafine micrometer strain sensors between layers
How to Simulate:
- EM stimulation near auriculotemporal nerve branch
- Mechanical impulse from speaker or actuator under gel
- Observe twitch/echo propagation time
📌 Math:
For conduction latency from EM field:
t=dvt = \frac{d}{v}t=vd
Where:
- ddd = distance to nerve branch (~1–5 cm)
- vvv = nerve conduction speed (~50–70 m/s for A-beta)
🧰 Final Toolkit (Phase 1 Testing Build List)
| Tool/Material | Purpose |
|---|---|
| Clear ballistic gel block with embedded hydrophone | Cavitation + internal pulse test |
| Acrylic plate with skin + tendon model | Resonance test for bone + fascia |
| Variable-resonance gel pads (10%, 15%, 20% gelatin) | Pressure wave transmission + Helmholtz response |
| Laser pointer + photodiode + mirror on knuckle | µ-vibration capture |
| Thermal camera with 0.05°C resolution | RF pressure / heating test |
| Piezo stack sensor with oscilloscope | Pulse detection, amplitude mapping |
| TENS unit | Nerve stimulation simulation |
| Low-cost SDR with directional antenna | Detect RF pulse presence (1.33 GHz etc.) |
🧠 1. Resonance-Based Interaction
Goal: Capture standing wave or resonance effects mimicking head or ear canal dynamics.
Simulation Plan:
- Material: Acrylic or polycarbonate for skull mimic; 3D-printed ear canal chamber.
- Geometry: Create a Helmholtz resonator tube connected to a cavity mimicking the auditory canal (e.g. ~2.5 cm long, 7 mm diameter).
- Sensor Placement:
- One piezo transducer at canal entrance
- One inside cavity wall (side-mounted)
- Optional: laser vibrometer on the outer wall for high-fidelity motion capture
- Input: Use RF or ultrasonic emitter aimed at the model.
- Measurement: Scan frequency range (100 Hz to 4 kHz), log resonance peaks.
💥 2. Cavitation / Microbubble Collapse
Goal: Recreate and measure cracking or popping that mimics knuckle-like cavitation.
Simulation Plan:
- Material: Gelatin (10%) or ballistic gel with injected microbubbles using syringe or soda carbonation; optionally use de-nucleated water in a chamber.
- Geometry: Cylindrical gel mold (3 cm thick, 8 cm wide) with embedded air pockets at 0.5 cm intervals.
- Sensor Placement:
- Contact mic or geophone beneath the gel block
- Accelerometer on surface
- Input: Mechanical plunger or pulsed ultrasound from side or bottom.
- Measurement: Detect cavitation-induced vibration spikes and decay rate.
📡 3. Electromechanical / RF-Induced Pressure
Goal: Simulate field-induced pressure pulses and thermoelastic expansion.
Simulation Plan:
- Material: Agar gel doped with 0.9% NaCl and carbon powder to simulate conductivity and absorption.
- Geometry: Flat 1 cm thick slab (ear-sized), with one embedded temperature sensor at center and two along sides.
- Sensor Placement:
- Infrared camera overhead
- Thermocouples embedded in gel
- Contact microphone under slab
- Input: Use SDR (e.g. HackRF or signal generator) to apply pulsed RF at 1.3 GHz or other suspect frequencies.
- Measurement: Look for small pressure pulses, thermal rise, or micro-expansion at timing intervals (e.g. every 3 seconds).
⚡ 4. Neuromechanical / Nerve-Fascia Coupling
Goal: Test tactile feedback response from skin/muscle interface layers mimicking nerve or tendon response.
Simulation Plan:
- Material: Dual-layer silicone sheet (~2 mm thick), separated by a layer of lubricant (e.g. glycerin gel) or embedded elastic thread.
- Geometry: Sheet tensioned over a small curved scaffold to mimic ear or temple area.
- Sensor Placement:
- Tactile pressure sensors between layers
- EMG skin electrodes outside layer for signal feedback
- Input: Mechanical or electrical stimulation (e.g. TENS pulse at surface)
- Measurement: Log reflex-like twitches, micropressure fluctuations, and delayed coupling effects.